Abstract
Objective: Sepsis is one of the most common clinical emergency and critical illness. The later stage of sepsis is prone to induce immunosuppression and results in secondary fatal infections. Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes the proliferation, differentiation and functional maturation of bone marrow hematopoietic stem cells, and has the function of enhancing immunity, so recombinant GM-CSF becomes an important direction for the treatment of sepsis-induced immunosuppression. However, the effect of recombinant GM-CSF on abnormal polymorphonuclear neutrophils (PMNs) in sepsis remains elusive. This study aimed to study the role of recombinant GM-CSF on the bactericidal ability of PMNs in septic mice, assessing its effect on the prognosis of secondary pneumonia.
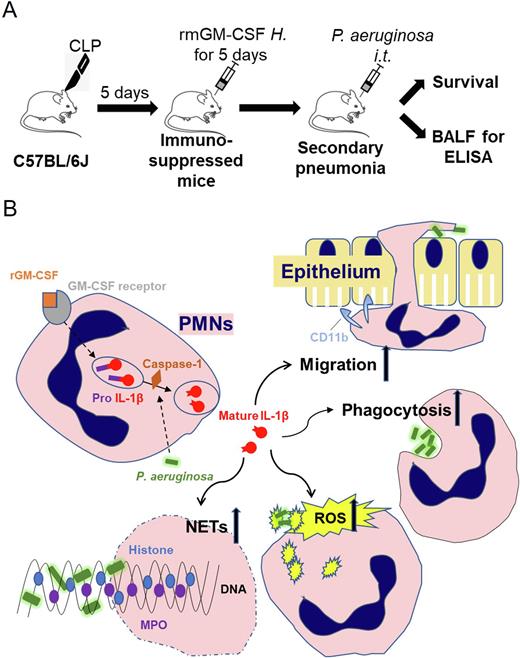
Methods: The C57BL/6J septic mouse model was induced by cecal ligation and puncture (CLP). Recombinant mouse GM-CSF (rmGM-CSF) was used in vivo when mice developed immunosuppression, then PMNs CD11b expression, reactive oxygen species (ROS), phagocytosis and neutrophil extracellular trap release (NETs) were detected by flow cytometry and fluoresce microplate reader. Pseudomonas aeruginosa pneumonia was induced by intratracheal injection after rmGM-CSF intervention in CLP mice, and the survival of mice were monitored. Meanwhile, PMNs from mouse peripheral blood were isolated for whole-transcriptomic sequencing. Combining with the results of bioinformatics analysis, anti-GM-CSF antibody and Caspase-1 inhibitor (Ac-YVAD-cmk) were treated into peripheral blood PMNs from septic patients in vitro, and then bactericidal ability of PMNs and intracellular IL-1β concentration were evaluated by flow cytometry and ELISA.
Results: 1. The septic mice were developed 5 days after CLP, which manifested as decreased lymphocytes in bone marrow, spleen and peripheral blood. The septic mice showed declined expression of MHC-II molecules and abnormal bactericidal function of PMNs in peripheral blood. 2. The expression of MHC-II in monocytes and the bactericidal function of PMNs were improved when septic mice were treated with rmGM-CSF in vivo. 3. RmGM-CSF improved the prognosis of secondary pneumonia in septic mice. 4. Percoll-isolated PMNs from septic patients were treated with rhGM-CSF in vitro. The expression of CD11b, ROS, phagocytosis and NETs release in PMNs were enhanced compared with those without rhGM-CSF treatments. 5. The whole-transcriptomic sequencing of mouse PMNs indicated that recombinant GM-CSF increased the expression of IL-1β in PMNs. 6. Caspase-1 is an important enzyme for the cleavage of pro form IL-1β into mature form. Ac-YVAD-cmk effectively inhibited the activation of IL-1β. treatments reduced the ROS, phagocytosis and release of NETs of PMNs, suggesting that rmGM-CSF enhanced the bactericidal ability of PMNs by IL-1β.
Conclusion: RmGM-CSF enhances the bactericidal function of PMNs in vivo, and improves the prognosis of secondary pneumonia in septic mice. In vitro experimental data showed rhGM-CSF strengthen PMNs bactericidal ability by stimulating active IL-1β expression.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal